Registration Process
The processes to register and obtain the required permits to conduct research with Controlled Substances and Dangerous Drugs may take time. Please, plan ahead at least two months before you can start a study.
See below for specific information about the process for Controlled Substances and Dangerous Drugs.
We have additional information under our FAQs for Controlled Substances and under our FAQs for Dangerous Drugs.
Controlled Substance Registration
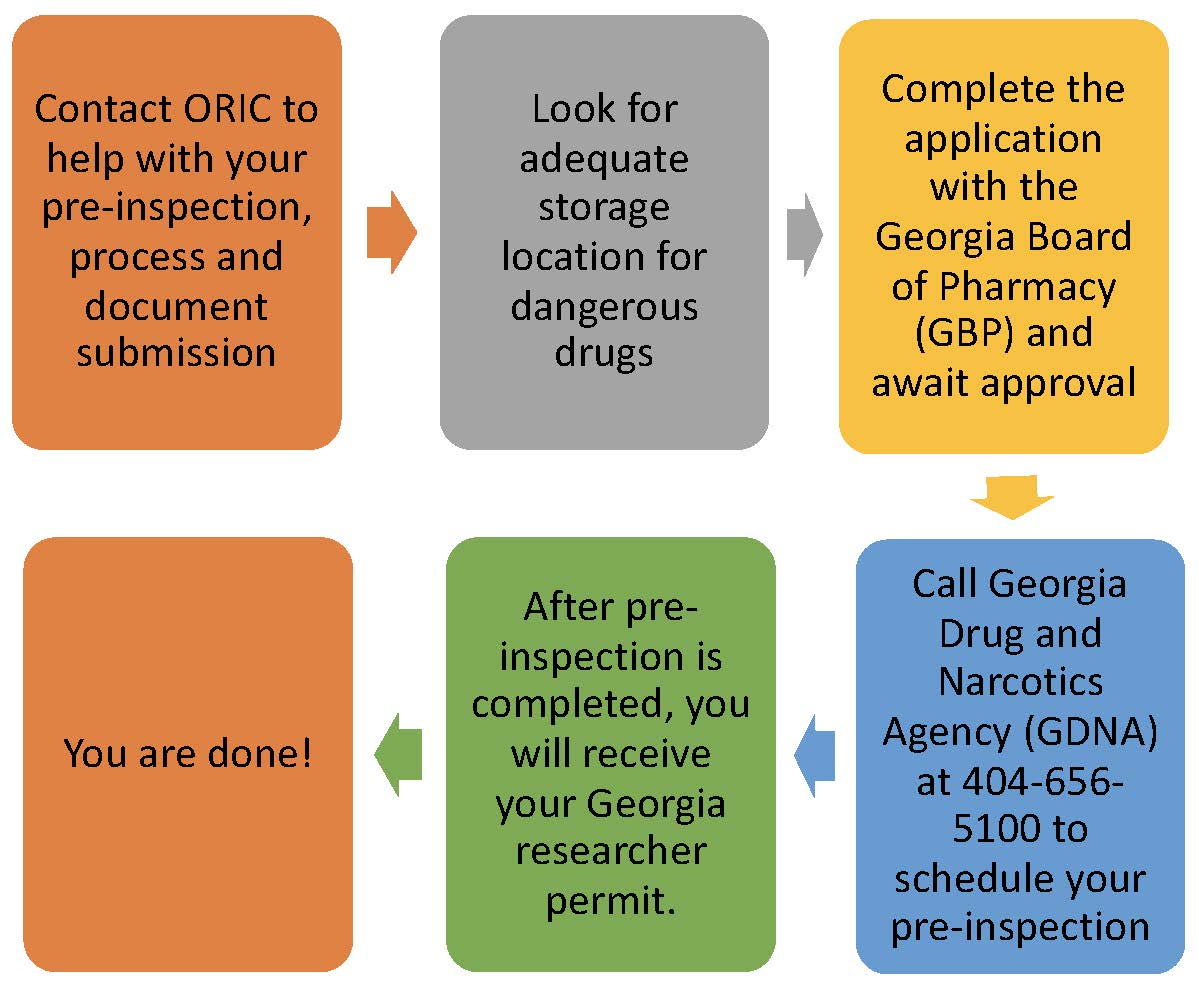
Look at our graphic below to have an understanding of the whole process with all the agencies. You will need to submit documentation with three oversight offices and go through a pre-inspection with GDNA and an inspection with DEA.
If all the documents and requirements submitted are correct, the whole process may take 8 weeks. Call us so we can assist you and prevent delays!
Registration and the documentation you need to submit would depend on if you are a licensed practitioner, the Schedule of the drug, and the type of research.
DEA Registration is site-specific. You are required to keep the drug stored at the same address that shows in your registration. In addition, DEA does not allow the drug to be used at a different address, so you must make arrangements to have the drug stored safely in the same building where the drug will be used. You will need a separate registration for each address if you conduct your research at multiple locations. DEA Registration for Schedule I Controlled Substances is also protocol specific, so if you change your research protocol, you must file a supplemental protocol with DEA. Permission is also required to increase the amount of Schedule I Controlled Substances used under the protocol. [See 21 CFR Section 1301.18].
See the following information to learn more.
If you are a licensed practitioner in Georgia (medical, veterinary, or another practitioner license)
- You will need a Georgia Researcher permit from the Georgia Board of Pharmacy (GBP). The Researcher license application is embedded in the Georgia Board of Pharmacy application form titled “Pharmacy Facility Application.” See this Sample (Go-by) to guide you in completing the form.
- You do not need a DEA registration unless you use a Schedule I drug. This Registration is not needed for schedules II to V.
- If using a Schedule I drug, please review this DEA Schedule I protocol checklist for more information on what to expect.
- You will need both a Georgia Research Permit from the GBP and a Research Registration from the DEA.
Note: DEA allows practitioners to dispense or conduct research with controlled substances in schedule II, III, IV, or V for human research if the applicant is authorized to dispense or conduct research concerning controlled substances under the laws of the State in which they practice. Georgia, however, requires a separate researcher permit to use controlled substances in research.
The registration process can be summarized as follows:
- You must apply for the Georgia permit first. The Researcher license is embedded in the Georgia Board of Pharmacy application form titled “Pharmacy Facility Application.” (Note: If you change to a different Controlled Substance schedule during the course of your research, you will need to file a new Georgia registration application. [See Pharmacy Facility Application, pg. 3, “Purpose of Applications” section, “Change in Schedule”]. Please note that the address that should be listed in the application is where you will be shipping and storing the drug.
- See this Sample (Go-by) to guide you in completing the form.
- The Georgia Drugs and Narcotics Agency (GDNA) must inspect your facility before granting the permit. They will check to ensure you have appropriate security, procedures, and documentation.
- Once you have the Georgia permit, you can apply for DEA registration. This can be done by completing the DEA Form 225 and submitting other needed documentation online.
- The DEA may rely on the GDNA inspection or come to inspect at its discretion.
- DEA Registration is site-specific. You are required to keep the drug stored at the same address that shows in your registration. In addition, DEA does not allow the drug to be used at a different address, so you must make arrangements to have the drug stored safely in the same building where the drug will be used. You will need a separate registration for each address if you conduct your research at multiple locations. DEA Registration for Schedule I Controlled Substances is also protocol specific, so if you change your research protocol, you must file a supplemental protocol with DEA. Permission is also required to increase the amount of Schedule I Controlled Substances used under the protocol. [See 21 CFR Section 1301.18].
Generally, the GDNA and DEA will look for the following security safeguards:
- Locks on rooms where Controlled Substances are stored
- Controlled Substances must be stored in securely locked cabinets or safes that cannot be easily moved and constructed so that forced entry is easily detected. Schedule I Controlled Substances must be kept in narcotics safe or similar container.
- There should be controlled access to the room in which the Controlled Substances are stored; a list must be maintained of persons with keys/codes to enter the room. Access to storage cabinets must be limited to only those authorized to work with Controlled Substances.
- Persons with access to Controlled Substances must be trained on applicable laws & procedures. They cannot have been convicted of a felony related to controlled substances or have had a DEA registration revoked.
- Schedule I and Schedule II must be kept separately from Schedule III to V Controlled Substances.
Emory conducts background checks and drug screens in new hires as part of their health assessment or separately if they work in a laboratory. Registrants are required to ensure that Form 3 is filled by anyone in the lab in contact with the drug.

Locked narcotics cabinets

Locked refrigerators or narcotics cabinets bolted to refrigerators
Go to the webpage for the Georgia Board of Pharmacy (GBP). The researcher license is embedded in the Georgia Board of Pharmacy application form titled “Pharmacy Facility Application”. ” Complete pages 3, 6, 7, 15, 16, and 17. Submission will require a brief resume and current photo (2x2 passport-style) and proof of U.S. citizenship or qualified alien status (i.e., lawful permanent resident, granted asylum, or admitted as a refugee). The initial application must be sent by U.S. Postal Service with a check. Thereafter renewals can be done online with a credit card.
See this Sample (Go-by) to guide you in completing the form.The GDNA will come to inspect your site before the GBP issues the Georgia Researcher Permit. To pass the inspection, you must ensure that you have appropriate processes and documentation in place for security, record-keeping, procurement, and disposal.
To find out what you need, go to our training page and take the training. Next, go to our Forms page to find the forms you need to put in place to keep track of Controlled Substances.
Use these forms and keep them up to date. Finally, familiarize yourself with our policy.
To apply, go to this link: https://apps.deadiversion.usdoj.gov/webforms2/spring/main?execution=e1s1
You can apply online by clicking “Complete DEA Form 225 Online.” Before completing Form 225, read the instructions on the page. Under the "Select Your Business Category," select "Researcher" under "Form 225". Under "Select One Business Activity," choose if you request a Schedule I or Schedule II registration, and click "Continue."
The DEA may rely on an inspection of your site by the Georgia Drugs and Narcotics Agency, or it may perform an additional inspection of your site.
DEA Registration is site-specific. You are required to keep the drug stored at the same address that shows in your registration. In addition, DEA does not allow the drug to be used at a different address, so you must make arrangements to have the drug stored safely in the same building where the drug will be used. You will need a separate registration for each address if you conduct your research at multiple locations. DEA Registration for Schedule I Controlled Substances is also protocol specific, so if you change your research protocol, you must file a supplemental protocol with DEA. Permission is also required to increase the amount of Schedule I Controlled Substances used under the protocol. [See 21 CFR Section 1301.18].
If you plan to conduct research with a Schedule I substance, please review this DEA Schedule I Researcher Pre-application Checklist for the requirements. The DEA requires several steps before the application that include:
- Georgia Pharmacy Board Approval
- IRB/IACUC/Institution approval
- Protocol creation as required by the federal regulations
Additional Considerations for CS Schedule I in Human Subjects Research
Use of Schedule 1 Controlled Substances in a clinical trial with human subjects requires obtaining a researcher permit from the GBP and registration with the DEA in addition to IRB approval. Investigators are advised to create an SOP for managing the Schedule 1 drug. Click here for an SOP Template. If an Emory investigator holds the Investigational New Drug application (IND), the investigator must first obtain this from the FDA. Click here for IND information, including IND submission forms and templates.
Investigators should consult with the Office of Research Integrity and Compliance (ORIC) to look at potential issues with state law and FDA research regulations. The Emory IRB will direct investigators to submit a completed Investigator Checklist for Use of Schedule 1 Controlled Substances to ORIC at oric@emory.edu for review of any research study using a Schedule 1 Controlled Substance. ORIC will perform a compliance review to ensure that processes are in place to meet state and federal requirements.
Registrants must order Controlled Substances for research through Emory’s Procurement Department https://www.finance.emory.edu/home/Procure%20and%20Pay/how_to_buy_in_the_marketplace/index_procure_pay_labresearchgoods.html.
- You must provide a copy of the Georgia Board of Pharmacy (GBP) Researcher permit or practitioner license and DEA registrations when ordering controlled substances.
A Registrant must order Schedule I and II Controlled Substances himself/herself, or alternatively, delegate a responsible person to perform orders by signing a Power of Attorney form. Schedule I and II Controlled Substances must be ordered using hardcopy DEA Form 222 or the DEA’s electronic Controlled Substances Ordering System (CSOS) process. To establish a CSOS account, the Registrant must contact McKesson, an Emory vendor of Controlled Substances, and complete the necessary registration form with DEA.
Generally, the GDNA and DEA will look for the following security safeguards:
- Locks on rooms where Controlled Substances are stored
- Controlled Substances must be stored in securely locked cabinets or safes that cannot be easily moved and constructed so that forced entry is easily detected. Schedule I Controlled Substances must be kept in narcotics safe or similar container.
- There should be controlled access to the room in which the Controlled Substances are stored; a list must be maintained of persons with keys/codes to enter the room. Access to storage cabinets must be limited to only those authorized to work with Controlled Substances.
- Persons with access to Controlled Substances must be trained on applicable laws & procedures. They cannot have been convicted of a felony related to controlled substances or have had a DEA registration revoked.
- Schedule I and Schedule II must be kept separately from Schedule III to V Controlled Substances.
Find this Pre-Registration Inspection Checklist to prepare for the inspection process.
Dangerous Drugs Registration
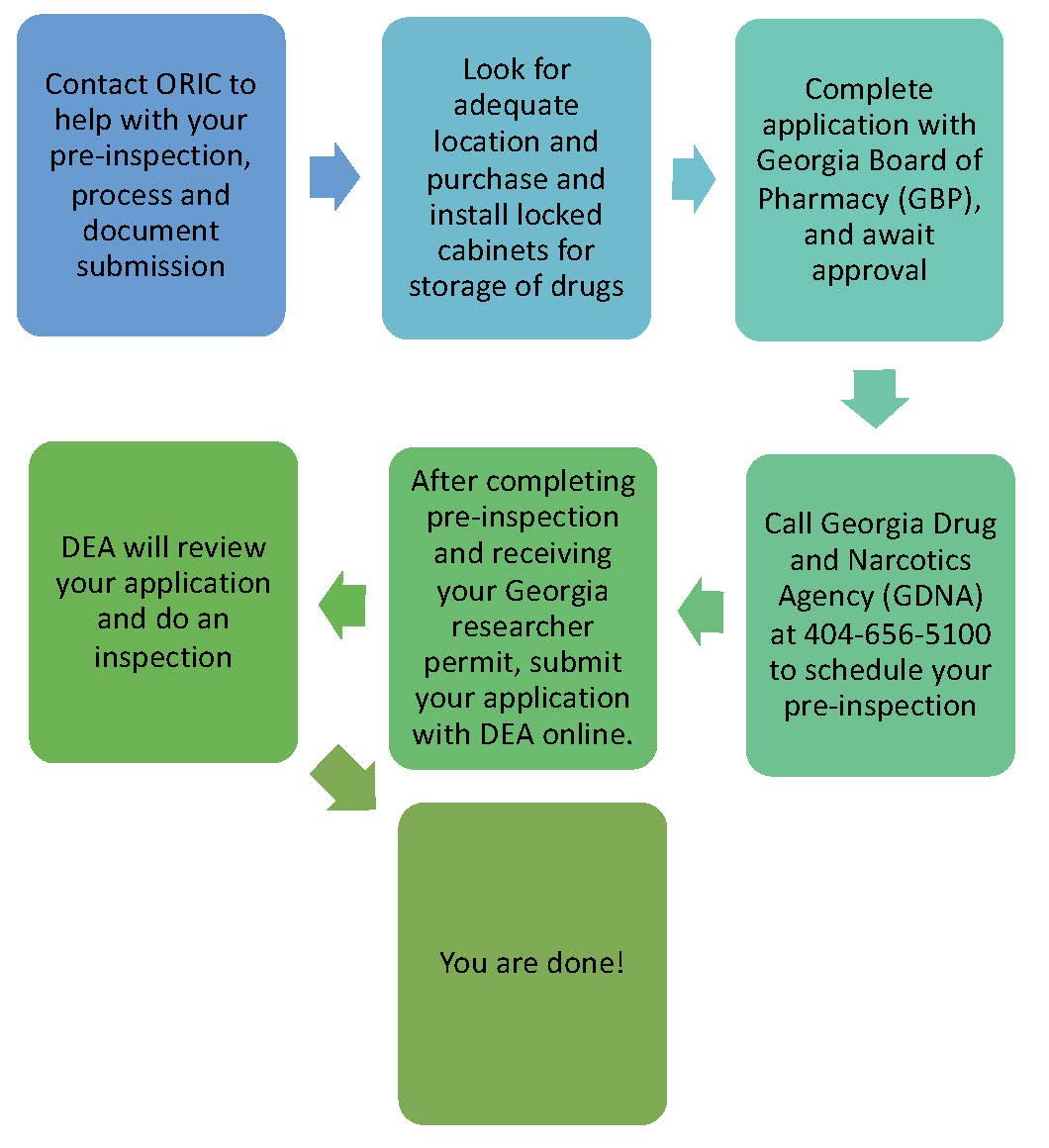
In Georgia, the use of Dangerous Drugs for Research is managed by the Georgia Board of Pharmacy (GBP) and the Georgia Drugs and Narcotics Agency (GDNA). You need to obtain the required permits before being able to order these drugs. The process also requires a pre-inspection, so you should plan ahead as the approval process may take up to 5 weeks. See below our graphic for details on this process.
The amount of time it takes to get a permit varies depending on individual circumstances and agency caseload. The Georgia Drugs and Narcotics Agency (GDNA) will need to inspect your facility before the permit is granted. They will check to make sure you have appropriate security, procedures, and documentation set up. Find this Pre-Registration Inspection Checklist to prepare for the inspection process.
You are required to keep the drug stored at the same address that shows in your registration. Ensure that you add all the locations where the drug will be stored in your registration.
Practitioners: You do not need additional permits if you are licensed as a practitioner (e.g., MD, DO, DDS, DVM, etc.) in the State of Georgia. You may obtain Dangerous Drugs for research using your practitioner license.
Non-Practitioners: If you are a researcher who is not licensed as a practitioner (e.g., a researcher with a Ph.D. degree but does not hold an MD degree), then you must obtain a Researcher Permit from the State of Georgia to be able to obtain Dangerous Drugs for research. You should provide Emory Purchasing a copy of the Georgia Board of Pharmacy (GBP) Researcher permit or practitioner license when you order the prescription drugs.
You are required to keep the drug stored at the same address that shows in your registration. Ensure that you add all the locations where the drug will be stored in your registration.
Go to the webpage for the Georgia Board of Pharmacy at http://gbp.georgia.gov/. Click on “Applications and Forms.” Click on the form entitled “Pharmacy Facility Application.” Complete pages 3, 6, 7, 15, 16, 17 and 18. Submission will require a brief resume and current photo (2x2 passport style) and proof of U.S. citizenship or qualified alien status (i.e., lawful permanent resident, granted asylum, or admitted as a refugee). The initial application must be sent by U.S. Postal Service with a check. After that, renewals can be done online with a credit card. See this Sample (Go-by) to guide you in completing the form.
The GDNA will come to inspect your site before the GBP issues the Georgia Researcher Permit. To pass the inspection, you must ensure that you have appropriate processes and documentation in place for security, record-keeping, procurement, and disposal.
To find out what you need, go to our training page and take the training. Next, go to our Forms page to find the forms you need to put in place to keep track of Controlled Substances.
Use these forms and keep them up to date. Finally, familiarize yourself with our policy.
Generally, the GDNA requires the same security safeguards for Dangerous Drugs as they and the DEA require for Controlled Substances. Here are the general requirements:
- The Dangerous Drugs must be stored in appropriate conditions, at temperatures that comport with their labeling requirements, and the storage area must be easy to clean and maintain.
- Dangerous Drugs must be stored in securely locked cabinets (or narcotics safes) where access is restricted to authorized personnel.
- Access to the cabinet (or safe) must be limited only to persons authorized to work with Dangerous Drugs.
- The area where the cabinet (or safe) is kept must be limited to a finite number of personnel.
- Persons with access to Dangerous Drugs must be trained on applicable laws & procedures.
- They cannot have been convicted of a felony related to Dangerous Drugs/Controlled Substances or have had a Dangerous Drugs/Controlled Substances license/registration/permit revoked.